Principle
The counterfeiting of medicines has increased with the sale of medicines on the Internet.
The World Health Organization (WHO) estimates that approximately 50% of the medicines sold on the Internet are falsified medicines (counterfeit medicines, unapproved medicines, etc.)
These medicines represent a major danger for your health. To avoid any risk, you should use websites that are authorised to sell medicines online.
The Act No. 1.518 of 23 December 2021 pertaining to the practice of pharmacy, more specifically articles 55 to 64, governs the sale of medicines via the Internet by a pharmacy
With regard to its enforcement, the Ministerial Decree No. 2016-555 of 12 September 2016 on the electronic sales of medicines (commerce électronique de médicaments), aims to provide a framework for requests for authorisation to create a website for the online sale of pharmaceutical products and the examination of these requests.
The medicines involved
The electronic sales of medicines is defined as economic activity in which a pharmacist offers or provides, remotely and by electronic means, the retail sale or dispensing to the public of medicines for human use and, to this effect, provides health information on line.
Medicines that can be sold on line are medicines that are not mandatory prescription drugs.
Internet sales of medicines for which a prescription is required are therefore forbidden.
Authorised websites
Pharmacies in the Principality
Pharmacists authorised to operate a pharmacy in the Principality can carry out electronic sales of medicines activity. This may only be undertaken via the pharmacy's website.
However, before undertaking this activity, such pharmacists must obtain authorisation to create a electronic sales of medicines website. The examination of the authorisation requests and any modifications are undertaken by the Department of Health Affairs.
Authorisations are issued by the Minister of State, after consideration by the Council of the Order of Pharmacists (Conseil de l’Ordre des Pharmaciens).
The named pharmacist is responsible for the content of the website he or she edits and the conditions in which the electronic sales of medicines activity is undertaken.
The cessation of the activity of the pharmacy shall automatically lead to the closure of its website.
Persons established in a State of the European Union
A natural or legal person established in amember State of the European Unionmay undertakeelectronic sales of medicines activity towards a person established in the Principality of Monaco, subject to certain conditions:
- They must only sell medicines that are not mandatory prescription drugs; these medicines must be authorised for sale on the market or registered in conformity with the legislation in force
- They must be legally qualified to sell to the public, including distance sales, in the State in which they are established
Recognising an authorised website
To help you identify authorised Monegasque websites, you are strongly advised to consult the list drawn up and updated bythe Council of the Order of Pharmacists, which will soon be available here.
Furthermore, Monegasque websites that are authorised for the online sale of medicines must include, a minima, the following information:
- The name of the pharmacy
- The surname and first names of the named pharmacist(s), or, if not applicable, of the assistant pharmacist(s) who have been delegated to operate the website
- The address of the pharmacy
- Phone number and fax number
- Details of the Ministerial Decree authorising the named pharmacist(s) to operate the abovementioned pharmacy
- The registration number of the entry in the register of trade and industry
- If relevant, the name and contact details of the website host
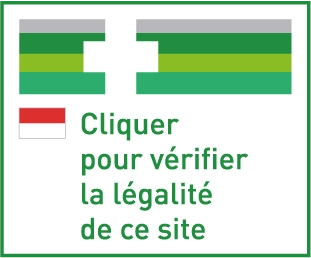
European Logo
Directive 2011/62 which is applicable in the Principality, introduces security measures for the online sale of medicines by providing a European legal framework.
This directive obliges electronic sales of medicines websites to display the following logo, which has been implemented throughout the Community, on every page of the website that deals with electronic sales of medicines (Regulation no. 699/2014).
By clicking on this logo, you can check whether a particular website appears on the list of websites that are authorised for electronic sales of medicines activity.
Risks related to medicines provided illegally on the Internet
The quality of medicines bought from an unauthorised website is not guaranteed, and falsified or counterfeit medicines can be offered for sale.
According to the WHO, falsified or counterfeit medicines can be found anywhere in the world.
The components of these medicines, including the active substances, are often of poor quality, falsified, in the wrong dosage or even absent, and therefore represent a serious threat to public health. They can consist of toxic mixtures of products, as well as ineffective preparations.
Sometimes it is difficult for both health professionals and patients to distinguish a falsified medicine from a genuine product.
In all cases, these medicines are of unknown origin and their composition is not reliable.
Counterfeit versions of all kinds of medicines exist, both specialised and generic.
See also
See also
Administrative contact
Le Puccini
48 boulevard d'Italie
MC 98000 MONACO
Opening hours :
from 8.30am to 5.30pm from Monday to Friday
Phone :
Fax :
Administrative contact
Le Puccini
48 boulevard d'Italie
MC 98000 MONACO
Opening hours :
from 8.30am to 5.30pm from Monday to Friday
Phone :
Fax :